Past President Argentine Lipid Society. Internal Medicine Doctor. School of Medicine Professor. Lipidologist. Atherosclerosis. CV Prevention.
-38.015717,-57.537636
Joined September 2009
- Tweets 16,762
- Following 2,049
- Followers 11,394
- Likes 23,106
👉 VESALIUS-CV: a line in the sand for earlier LDL-C lowering
👆 Bottom line: In high-risk adults without prior MI or stroke, pushing LDL-C to physiologic levels (~30–50 mg/dL) prevents first events—with no safety penalty. This isn’t a “brand story”; it’s biology. Treat the under-recognized, undertreated cohort earlier, harder, and lower. Causality reaffirmed.
What VESALIUS-CV did.
👆 12,000+ patients with atherosclerosis or high-risk diabetes (LDL-C ≥90 mg/dL, on optimized therapy) randomized to PCSK9 inhibition vs placebo, ~4.6 years follow-up.
👆 Primary outcomes: 3-point MACE (CHD death, MI, stroke) and 4-point MACE (+ revascularization).
Results: HR 0.75 and 0.81; MI −36%. No safety signal at median LDL-C ≈45 mg/dL.
👆 Why it matters (and for whom).
Not post-MI patients—these are our everyday high-risk adults: atherosclerosis without prior events or long-standing diabetes. Historically in a gray zone—recognized risk but seldom pushed to physiologic LDL targets. VESALIUS-CV proves that doing so prevents first events. That’s a practice change.
Beyond brands: proof of concept.
👆 Benefit mirrors the depth and duration of LDL-C lowering, as CTT and cross-mechanism data show. Shorter PCSK9 trials underestimated the long-term gain; extended follow-up here exposes the true magnitude. The molecule is incidental; the biology, universal.
👆 Re-stating the obvious: LDL causes ASCVD. Statins, genetics (PCSK9, HMGCR), and nonstatins all align: lower ApoB/LDL-C → fewer events. VESALIUS-CV extends that to high-risk, event-free patients—showing the curve moves with LDL-C down to ~45 mg/dL, safely.
👆 Physiologic targets are safe.
Humans start life at 30–50 mg/dL; disease begins as LDL climbs. VESALIUS-CV confirms long-term safety at ~45 mg/dL—another proof that physiologic levels are protective.
👆 Who benefited?
Both: those with atherosclerosis and those with high-risk diabetes (~⅓ of cohort). Translation: don’t wait for the first infarct. Prevent it.
👆 On Monday morning:
Screen and stage risk early (CAC, plaque, PAD, diabetes).
Treat to physiologic LDL-C (30–50 mg/dL, ApoB aligned).
Stop accepting 90–120 mg/dL in “primary” high-risk.
Start earlier, stay longer—benefits compound with time.
👆 Clinic one-liner:
LDL causes ASCVD. Treat high-risk, event-naïve patients to physiologic LDL-C (30–50 mg/dL)—regardless of drug class—and you’ll prevent first events
🔗 nejm.org/doi/full/10.1056/NE…
@society_eas
🚨 AHA 2025 | CORALreef Lipids Trial
A true game changer in lipid management.
💊 Enliciditide (MK-0616) — the first oral PCSK9 inhibitor — achieved a 57% LDL-C reduction at week 24 and −50% sustained at week 52 vs placebo (p<0.001).
👥 Phase 3 trial, 2,912 patients with ASCVD or at high risk, across 14 countries, with excellent safety profile.
👉Takeaway: efficacy and safety comparable to injectable PCSK9 inhibitors — but in a once-daily oral tablet.
☝️A new era in PCSK9 inhibition and cardiovascular prevention.
@society_eas
#AHA2025
Mortality here is hard to show—low event rates, short horizon. But a 25% MACE reduction on top of statins is the signal. This is still about LDL-C/apoB lowering. Mortality follows with time and number
The question here I think is not that lower LDL is better, it is. The question is value and work of being a patient.
You take costly injections for 4.6 years and the MI reduction is not enough to reduce CV death.
Questions the value of MI as a surrogate. I dunno. #AHA25
🚨 Breaking from #AHA25 & @NEJM
The first in vivo CRISPR–Cas9 gene-editing trial targeting ANGPTL3 (CTX310) shows robust lipid reductions after a single infusion:
•⬇️ 80% ANGPTL3,
•⬇️ 49% LDL-C,
•⬇️ 55% triglycerides,
with no dose-limiting toxicity.
A potential once-in-a-lifetime therapy to permanently lower atherogenic lipoproteins.
@society_eas
nejm.org/doi/full/10.1056/NE…
🚨 New breakthrough in prevention!
👉In the VESALIUS‑CV trial, Evolocumab added to high-intensity statin therapy reduced major adverse cardiovascular events (MACE) by 25% (HR 0.75; 95% CI 0.65-0.86) over ~4.6 years in patients who had no prior MI or stroke.
👉Absolute risk reductions:
• ~1.8% for death from coronary disease, MI, or stroke.
• ~2.8% when adding ischemia-driven revascularisation.
👉LDL-C fell ~55% to ~45 mg/dL—highlighting once again that “lower is better”, even before a first event.
☝️What this means for clinical practice:
• This is the first PCSK9 inhibitor trial showing benefit in a “pre–first event” high-risk population, not just secondary prevention.
• Identifying high-risk patients (e.g., established atherosclerosis, high-risk diabetes) before MI or stroke is crucial.
• This supports aggressive LDL-C lowering to ~40 mg/dL in selected patients—even without prior event history.
☝️Take-home: For the right high-risk patient, adding evolocumab before the first major cardiovascular event can be a game-changer. Traditional prevention meets cutting-edge therapy.
@society_eas
nejm.org/doi/full/10.1056/NE…
💉Antibody-based therapies: redefining cholesterol management
👉Statins remain the foundation of lipid-lowering therapy, yet many patients still fail to reach LDL-C goals. Antibody-based therapeutics are changing that landscape.
👉Key highlights:
1️⃣ 💉Alirocumab and Evolocumab — established PCSK9 inhibitors proven to reduce LDL-C by ~60% and lower cardiovascular events.
2️⃣ 🇨🇳 Four new PCSK9 monoclonal antibodies (Tafolecimab, Ongericimab, Ebronucimab, Recaticimab) recently approved in China, offering longer dosing intervals and robust efficacy.
3️⃣ 💊Emerging alternatives: oral PCSK9 inhibitors (e.g., Enlicitide MK-0616, Laroprovstat AZD0780), siRNA (Inclisiran), and fusion proteins (Lerodalcibep).
4️⃣ 💉Evinacumab — ANGPTL3 inhibitor effective in homozygous FH, acting independently of LDL receptors.
5️⃣ 🧬Next frontier: CRISPR-based and siRNA therapies targeting PCSK9 and ANGPTL3 may permanently reshape lipid control.
☝️Bottom line:
Antibody-based lipid therapies are moving from niche to mainstream — combining potent LDL-C reduction with extended dosing and precision targeting for residual risk.
🔗 Open Access doi.org/10.2147/btt.s500456
@society_eas
@DovePress
🧠💔Lower LDL-C, Lower Risk — Safely
New analysis from FOURIER & FOURIER-OLE (Monguillon et al., Circulation 2025):
In patients with prior ischemic stroke, achieving LDL-C <40 mg/dL led to a continuous reduction in MACE and recurrent stroke,
👉 without any increase in hemorrhagic stroke.
🔹 5,291 stroke patients
🔹 Follow-up up to 7 years
🔹 Stepwise reduction in cardiovascular and ischemic events with lower LDL-C
🔹 Hemorrhagic strokes: rare and unrelated to LDL-C
☝️Conclusion: Very low LDL-C levels (<40 mg/dL) are both effective and safe for secondary prevention after stroke — confirming that “the lower, the better” also applies to the brain.
🔗 ahajournals.org/doi/pdf/10.1…
@society_eas
@CircAHA
An unforgettable academic experience in Copenhagen 🇩🇰
🙏🏻I’m deeply grateful to Professors Anne Tybjaerg-Hansen and Børge Nordestgaard for the kind invitation to serve as a member of the PhD evaluation committee at the University of Copenhagen.
🙌Congratulations to Dr. Lærke Kristine Kyhl for her outstanding defense and successful completion of her PhD thesis: “Metabolic dysfunction-associated steatotic liver disease, cardiometabolic risk factors, and cardiovascular disease.”
😍It was truly inspiring to witness such a rigorous and elegant presentation, reflecting years of dedicated research.
🤯Beyond the academic excellence, it was a unique opportunity to explore Herlev Hospital, a remarkable center of knowledge and innovation — the birthplace of many breakthroughs in dyslipidemia, atherosclerosis, and cardiovascular prevention.
🤩Grateful to have shared this memorable moment surrounded by colleagues, mentors, and friends.
@society_eas
@UFASTA
@BNordestgaard
@uni_copenhagen
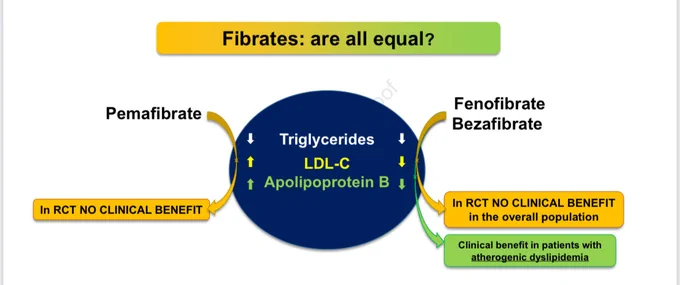
👉Not all fibrates are created equal — and it’s time we stop pretending they are.
1️⃣ Decades of data, one clear message: fenofibrate stands alone in lowering apoB, non-HDL-C, and triglycerides — the true drivers of residual cardiovascular risk.
2️⃣ Pemafibrate? Potent in the lab, powerless in outcomes.
3️⃣ Gemfibrozil? Obsolete — interactions and limited efficacy.
4️⃣ Bezafibrate? Inconsistent.
5️⃣ Fenofibrate remains the only fibrate with hard-endpoint data, microvascular protection, and long-term safety in combination with statins.
☝️Fibrates are not interchangeable — biology and outcomes both prove it.
💡 It’s time to rethink how we treat atherogenic dyslipidemia.
🔗 atherosclerosis-journal.com/…
@society_eas
@ATHjournal
👉Pushing the limits of LDLc: Emerging paradigms in CV risk reduction
1️⃣ Modern guidelines now target LDL-C <55 mg/dL, and even <40 mg/dL for the highest-risk patients.
2️⃣ Evidence from FOURIER, ODYSSEY, and IMPROVE-IT confirms that the lower, the better — with no safety concerns, even below 25 mg/dL.
3️⃣ Genetic data (PCSK9 LoF, APOB, ANGPTL3) reinforce that lifelong low LDL-C protects against ASCVD.
4️⃣ Emerging therapies — PCSK9 inhibitors, inclisiran, bempedoic acid, ANGPTL3 inhibitors — make these targets achievable.
5️⃣ Cognitive, endocrine, and hemorrhagic risks remain none at very low LDL-C.
☝️The real challenge is no longer how low can we go, but how many actually get there.
🔓Open Access
sciencedirect.com/science/ar…
@society_eas
👉New in ATVB (Nov 2025)
🧪Multiplex Apolipoprotein Panel Boosts Cardiovascular Risk Prediction & Precision Therapy
👥 In >11,800 post-ACS 💔patients from ODYSSEY OUTCOMES, a 9-plex mass-spectrometry apolipoprotein paneloutperformed the standard lipid profile in predicting:
•🔹 Major adverse cardiovascular events (MACE) (AUC 0.65 vs 0.58)
•🔹 All-cause death (AUC 0.70 vs 0.60)
💡 The panel also identified which patients benefit most from PCSK9 inhibition (alirocumab) — paving the way for precision lipidology.
🧩 Key components: Apo(a), ApoA-I, ApoA-II, ApoA-IV, ApoB, ApoC-I, ApoC-II, ApoC-III, ApoE.
📉 Alirocumab reduced Apo(a), ApoB, and ApoC isoforms by >30–50%.
☝️ Bottom line:
Integrated apolipoprotein profiling refines risk stratification and optimizes treatment allocation — a step forward in personalized cardiovascular medicine.
🔓Open Access
🔗 ahajournals.org/doi/10.1161/…
@society_eas
@atvbahajournals
👉Metabolic dysfunction-associated steatotic liver disease, cardiometabolic risk factors, and CVD
🫀MASLD: not just a liver disease
👉Nearly 40% of adults worldwide have metabolic dysfunction–associated steatotic liver disease.
But what kills most of them isn’t liver failure — it’s cardiovascular disease.
🤔The link?
Too much fat in the liver, too much VLDL & remnant cholesterol in the blood.
The same dyslipidemia that feeds atherosclerosis.
☝️MASLD is a cardiometabolic disease — what happens in the liver doesn’t stay in the liver.
🔗 Open Access atherosclerosis-journal.com/…
@society_eas
@ATHjournal
@BNordestgaard
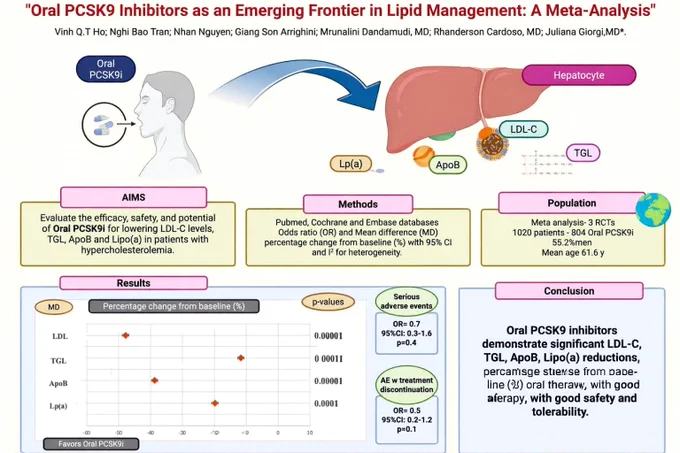
👉Oral PCSK9 Inhibitors: A New Frontier in Lipid Management
👆A meta-analysis including >1,000 patients shows that oral PCSK9 inhibitors reduce key atherogenic lipoproteins by a clinically meaningful magnitude:
🔹 LDL-C ↓ 47.8%
🔹 ApoB ↓ 38.7%
🔹 Lp(a) ↓ 19.8%
🔹 Triglycerides ↓ 11.6%
Importantly, these agents demonstrated no increase in serious adverse events versus placebo.
💊 Oral PCSK9 inhibitors could transform lipid management — combining potent LDL-lowering efficacy with the convenience of oral dosing, potentially improving accessibility and adherence for patients with ASCVD or familial hypercholesterolemia.
🔗doi.org/10.1016/j.jacl.2025.…
@society_eas
@nationallipid
The evolution of plasma cholesterol: Direct utility or a ‘‘spandrel” of hepatic lipid metabolism?
🧬 LDL: Evolution’s Beautiful Mistake
What if “LDL” was never meant to exist?
☝️LDL is not a product of evolutionary design, but an accident of hepatic lipid metabolism — a spandrel, a non-adaptive by-product that emerged when the liver evolved to detoxify fatty acids through VLDL secretion.
☝️In this view, LDL is the collateral damage of a system built to protect us from lipotoxicity. Cholesterol didn’t evolve to serve a purpose in the plasma — it simply hitched a ride on a transport system that couldn’t get rid of it.
👉The irony is striking: what keeps us alive (efficient fat transport) also slowly kills us (atherosclerosis). Evolution doesn’t optimize for longevity — it optimizes for survival until reproduction. LDL might just be the price we pay for being multicellular, energy-hungry organisms.
sciencedirect.com/science/ar…
@society_eas
@Drlipid
@PeterAttiaMD
@JohnKastelein
👉Challenges in the Choice of Nonstatin Medications for LDL-C Lowering for Cardiovascular Risk Reduction
👉New review in @JAHA_AHAJournals
Myerson et al. (2025) analyze the challenges in choosing non-statin LDL-C-lowering therapies for ASCVD prevention.
🔹 Statins remain first-line, but many patients need add-on or alternative options.
🔹 Ezetimibe, bempedoic acid, PCSK9 mAbs, and inclisiran expand the therapeutic armamentarium.
🔹 Up to 60% additional LDL-C reduction and proven outcome benefits in selected trials.
🔹 Emphasis on personalized lipid management balancing efficacy, adherence, and cost.
🔓Open Access: ahajournals.org/doi/10.1161/…
@society_eas
@American_Heart
🙌🏼 State-of-the-art review:
👉Triglyceride-rich lipoproteins, remnants and atherosclerotic cardiovascular disease: What we know and what we need to know
☝️This comprehensive review dissects the biological complexity and clinical significance of triglyceride-rich lipoproteins (TRLs) and their remnants in the context of atherosclerotic cardiovascular disease (ASCVD).
☝️Genetic and mechanistic data confirm that TRL remnants are more atherogenic per particle than LDL, driven by enhanced arterial wall retention, pro-inflammatory lipid cargo, and endothelial dysfunction. Yet, clinical trials lowering triglycerides have yielded inconsistent cardiovascular benefits, reflecting the structural and metabolic heterogeneity of TRL particles and their dynamic remodeling into cholesterol-enriched forms.
☝️The authors emphasize critical unmet needs:
•Identification of TRL subspecies and bioactive lipid components most responsible for vascular injury.
•Development of refined biomarkers of TRL-related atherogenicity.
•Adoption of precision medicine strategies targeting individual TRL profiles.
☝️Ultimately, advancing TRL science will require integrating lipidomics, genetics, and systems biology to close the gap between triglyceride lowering and tangible ASCVD risk reduction.
@society_eas
@ATHjournal
🔗atherosclerosis-journal.com/…
🙌 Many thanks to @drdargaray , and Alexandra Arias Mendoza for the invitation to another edition of the @congresoC3.
👏 An incredible turnout, combined with an outstanding scientific program and flawless organization.
A truly unmissable event — year after year, it keeps raising the bar in every way.
🙌Muchas gracias Diego Araiza Garaygordobil, MD PhD y Alexandra Arias Mendoza por la invitación a una nueva edición del Congreso C3.
👏Increíble convocatoria, sumado a un programa científico inmejorable y una organización impecable.
Un evento para no perdérselo, año tras año un escalón hacia arriba en todos los sentidos.
😍 Honored to participate in the Cardiovascular Congress of @fcardioinfantil in Bogotá, where I had the privilege of sharing the stage with one of my mentors and long-time inspiration in lipidology, Dr. Thomas Dayspring (@Drlipid).
🙌🏼 His knowledge and teaching on lipid and lipoprotein complexities have guided generations of clinicians and researchers, and it was truly special to join this congress alongside him.
🤩 Grateful to the organizers for this outstanding scientific program and for the opportunity to contribute to such an enriching exchange.
Many thanks to FUNDACION CARDIOINFANTIL of Bogota Columbia, for the invitation and high honor to lecture virtually at their Cardiovascular Congress today on the topic of Lipid and Lipoprotein complexities. It was a pleasure to join with my long-time Argentinian friend and lipidology colleague @drpablocorral . And if you are wondering, yo no hablo Espanol 😶 @nationallipid @society_eas @ASPCardio @escardio @atherosociety @FamilyHeartFdn @foundationofnla
🙌 Muchas gracias a toda la comunidad de @fcardioinfantil por la invitación.
Un placer poder participar junto a @Drlipid
👉New Insights from CLEAR Outcomes on Bempedoic Acid and Gout
A recent post hoc analysis of the CLEAR Outcomes trial provides important clinical insights into the relationship between bempedoic acid, uric acid levels, and gout incidence.
🔑 Key findings:
•Bempedoic acid reduced LDL-C by ~21% and MACE-4 by 13%, reinforcing its cardiovascular benefit in statin-intolerant patients.
•A modest rise in uric acid (~0.8 mg/dL) was observed, with slightly higher rates of gout versus placebo.
•Uric acid–lowering therapies (mainly allopurinol) significantly mitigated the risk of gout, especially in patients with baseline hyperuricemia or prior gout.
•Importantly, cardiovascular benefit was consistent in patients with or without prior gout history.
☝️Clinical implication: Episodes of gout should not preclude the use of this potentially cardioprotective therapy. With appropriate monitoring and urate-lowering therapy, bempedoic acid remains a valuable option for patients intolerant to statins and at high CV risk.
👉 Full article: sciencedirect.com/science/ar…
@society_eas
@ProfKausikRay